IDT’s GMP oligos and services prioritize your needs. We exceed expectations with expert support and dedication to advancing biology and medicine. From concept to final product, every step meets your specifications. Our streamlined options and GMP expertise help you find the best solutions for success.
GET STARTEDIDT’s expertise in GMP oligo manufacturing allows us to help you through every step of your assay development, from discovery to commercialization. We offer a streamlined portfolio to aid in GMP product selection, order processing, and transparent pricing.

Unlock the full potential of your research with IDT's GMP oligo expertise. Schedule a consultation today to explore tailored solutions for your assay development needs.
IDT's commitment to quality and precision is evident in our ISO 13485:2016 certified services:
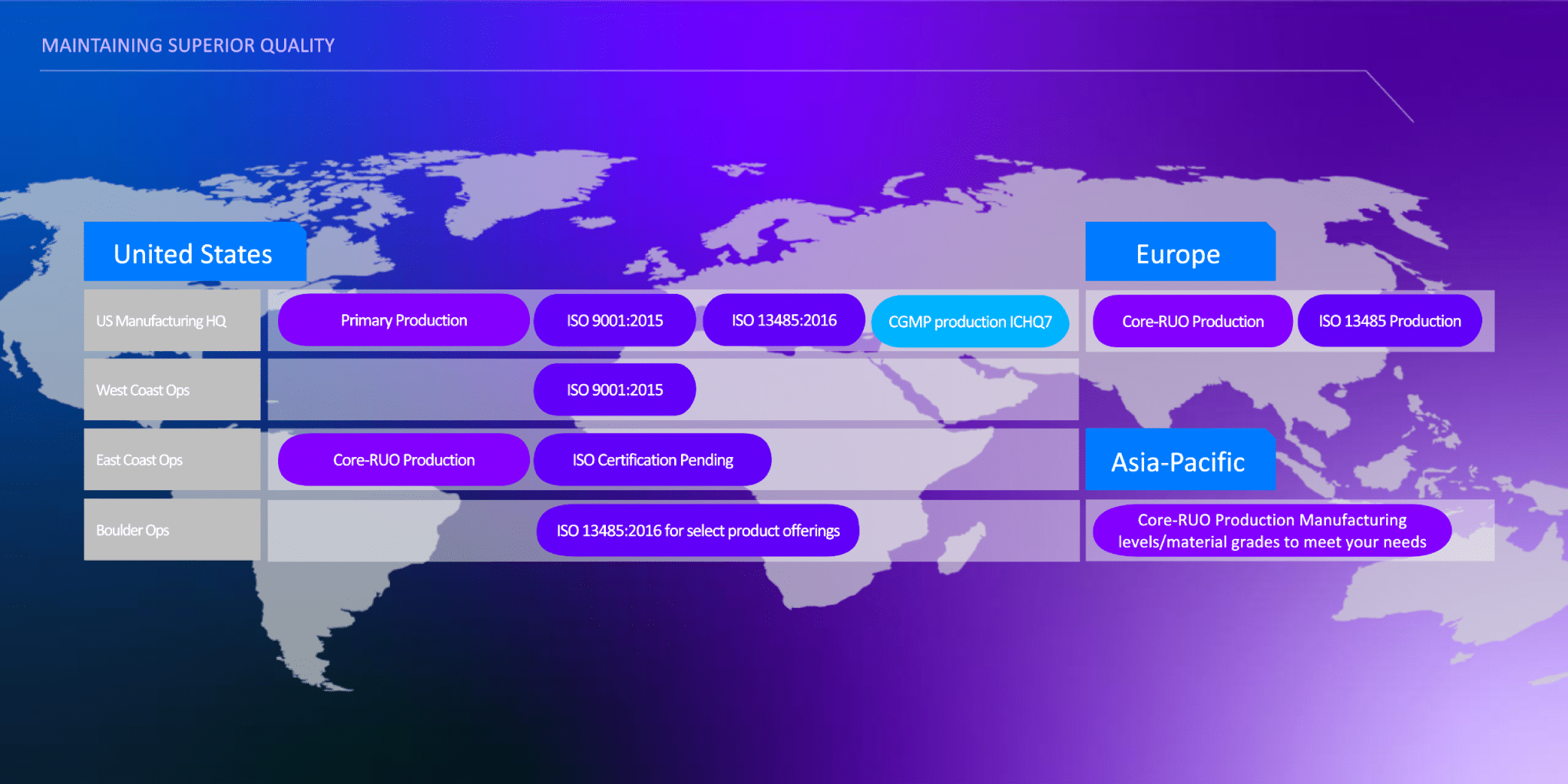
GMP refers to products manufactured under ISO 13485: 2016 QMS. Purchaser is solely responsible for all decisions regarding the use of these products and any associated regulatory or legal obligations for their legal marketing.