xGen™ Methylation-Sequencing DNA Library Preparation Kit
Comprehensive methylome coverage from low-input research samples
xGen Methyl-Seq Library Prep Kit utilizes Adaptase™ technology that efficiently captures bisulfite-converted ssDNA molecules into library molecules for epigenetic research studies. The resultant libraries represent uniform and comprehensive genome coverage.
xGen NGS—made reliable.
Ordering
- Comprehensive sample representation—uniform methylome genome coverage by whole genome bisulfite sequencing (WGBS)
- Low bias library preparation from low input amounts—supports inputs down to single cell
- High multiplex capability—pre-plated UDI primers enable multiplexing of up to 1536 samples
- Compatible with xGen Normalase™ technology—streamlines normalization of libraries enzymatically for a single library pool
- Powered by xGen Adaptase technology—template independent adapter attachment reduces workflow time while generating a less biased library
- Simple, 2-hour workflow
- Workflow design for easy automation
Senior Genomics Technologist
Queensland University of Technology
After prepping 50 samples with a DNA kit from another vendor and getting poor-quality libraries, I tried IDT's xGen EZ DNA prep kit on the exact same samples and got perfect libraries. We have also switched to this kit for plant samples that have failed with other vendors kits and everyone has been happy with the data. I have had similar experiences with methylation libraries that worked better with IDT and RNA. Overall, the kits are easy to use and give great results and we have switched several of our preps over to them.
Product details
DNA methylation is the most widely studied epigenetic modification. DNA methylation occurs primarily on cytosine bases in the CpG dinucleotide motif in most mammalian cells, and across all cytosine contexts in plant genomes. DNA methylation is often associated with the regulation of gene expression through its presence or absence in CpG islands among gene promoter regions, and these methylation patterns can be used to identify different cell types and disease states [1].
IDT offers two products for methylation sequencing:
- xGen Methyl-Seq DNA Library Prep Kit for whole genome and targeted methylation sequencing
- xGen Adaptase Module for single cell methylation sequencing
Some common applications of methyl-seq include:
- Whole genome bisulfite sequencing (WGBS) research
- Identifying genome-wide methylation of cell-free (cfDNA) in research studies
- Hybridization capture for targeted methylation studies
- Bisulfite-converted DNA enriched by MeDIO, chromatin immunoprecipitation (ChIP), or other research methods
xGen Methyl-Seq Library Prep Kits
The xGen Methyl-Seq DNA Library Prep Kit can be used for WGBS and targeted bisulfite sequencing with the addition of an xGen Custom Hyb Panel. The kit’s post-bisulfite library preparation workflow offers an efficient library preparation method. The kit is compatible with low input quantities such as cfDNA for biomarker research studies. The xGen Methyl-Seq DNA Library Prep Kit is also compatible with bisulfite-converted DNA samples enriched by ChIP or other methods as well as ancient DNA samples that may be deaminated.
2-hour workflow for bisulfite-converted samples
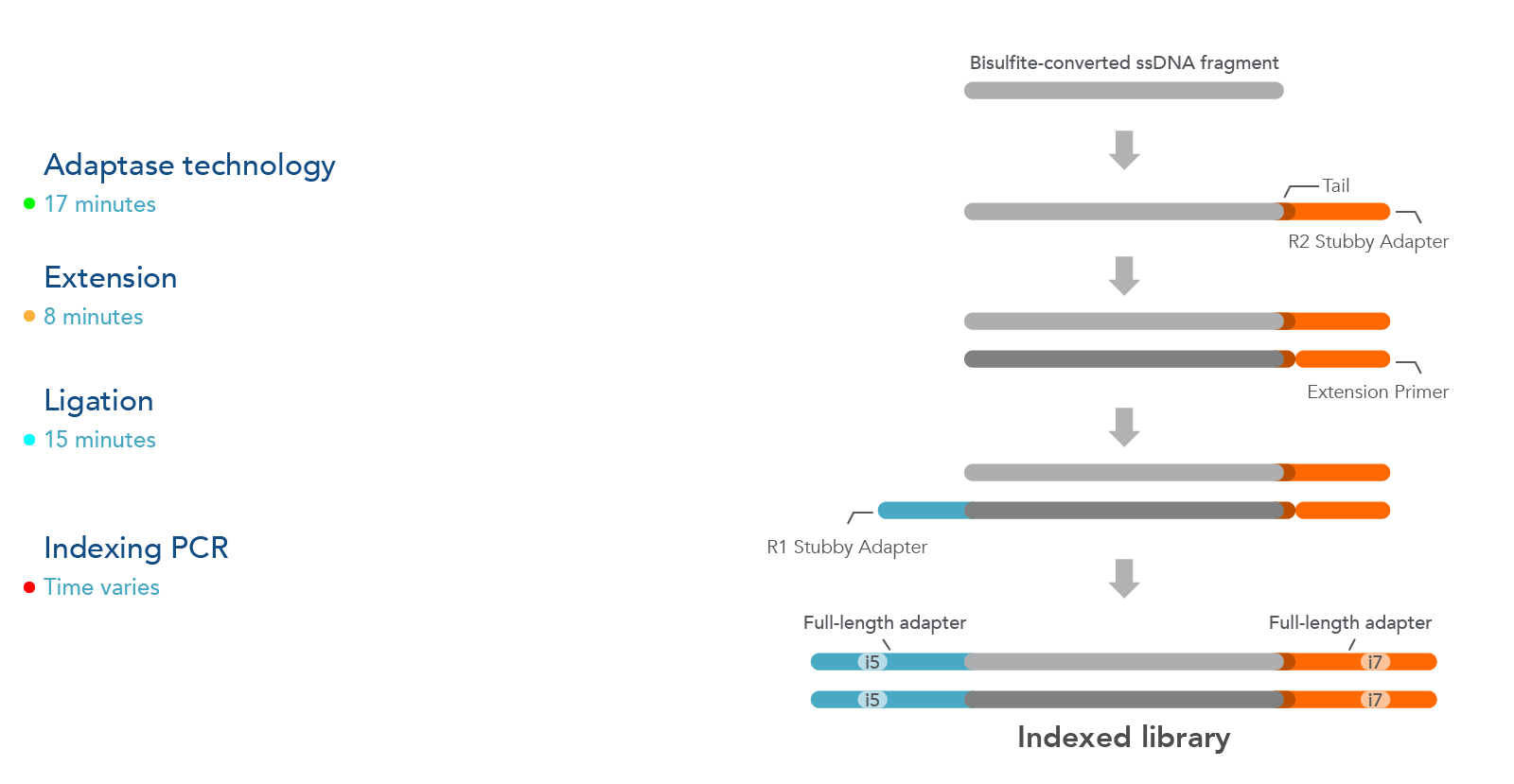
The xGen Methyl-Seq DNA Library Prep Kit workflow maximizes DNA recovery through a post-bisulfite library preparation, utilizing an efficient adapter attachment that is compatible with single-stranded, bisulfite-converted DNA (Figure 1). Library complexity from this kit is significantly greater than those from methods that bisulfite convert after library construction (see Table 2).
Additionally, the template-independent adapter attachment chemistry of the xGen Methyl-Seq DNA Library Prep Kit provides a more complete, less biased library as observed from comprehensive methylome coverage by WGBS (see Table 2).
Figure 1. The xGen Methyl-Seq DNA Library Prep Kit workflow constructs libraries from single-stranded, bisulfite-converted DNA fragments. The Adaptase step simultaneously performs end repair, tailing, and ligation of
the R2 Stubby Adapter to the 3’ end of each fragment. The extension step produces a uracil-free strand and the ligation step adds R1 Stubby Adapter to the uracil-free strand. Indexing PCR increases library yield and incorporates
full-length adapters with sample-specific index sequences.
Table 1. Specifications and features of the xGen Methyl-Seq DNA Library Prep Kit.
| Feature | xGen Methyl-Seq DNA Library Prep Kit |
|---|---|
| Sample types | gDNA, FFPE, cfDNA |
| Input DNA amount | 100 pg to 100 ng |
| Indexing compatibility | CDI primers up to 96-plex, with or without Normalase technology UDI primers up to 1536-plex, with or without Normalase technology |
| System compatibility and multiplexing format | Illumina® sequencing instruments |
| Workflow compatibility | Manual & automated. For list of liquid handlers and scripts, please inquire. |
xGen Adaptase Module for single cell methylation sequencing
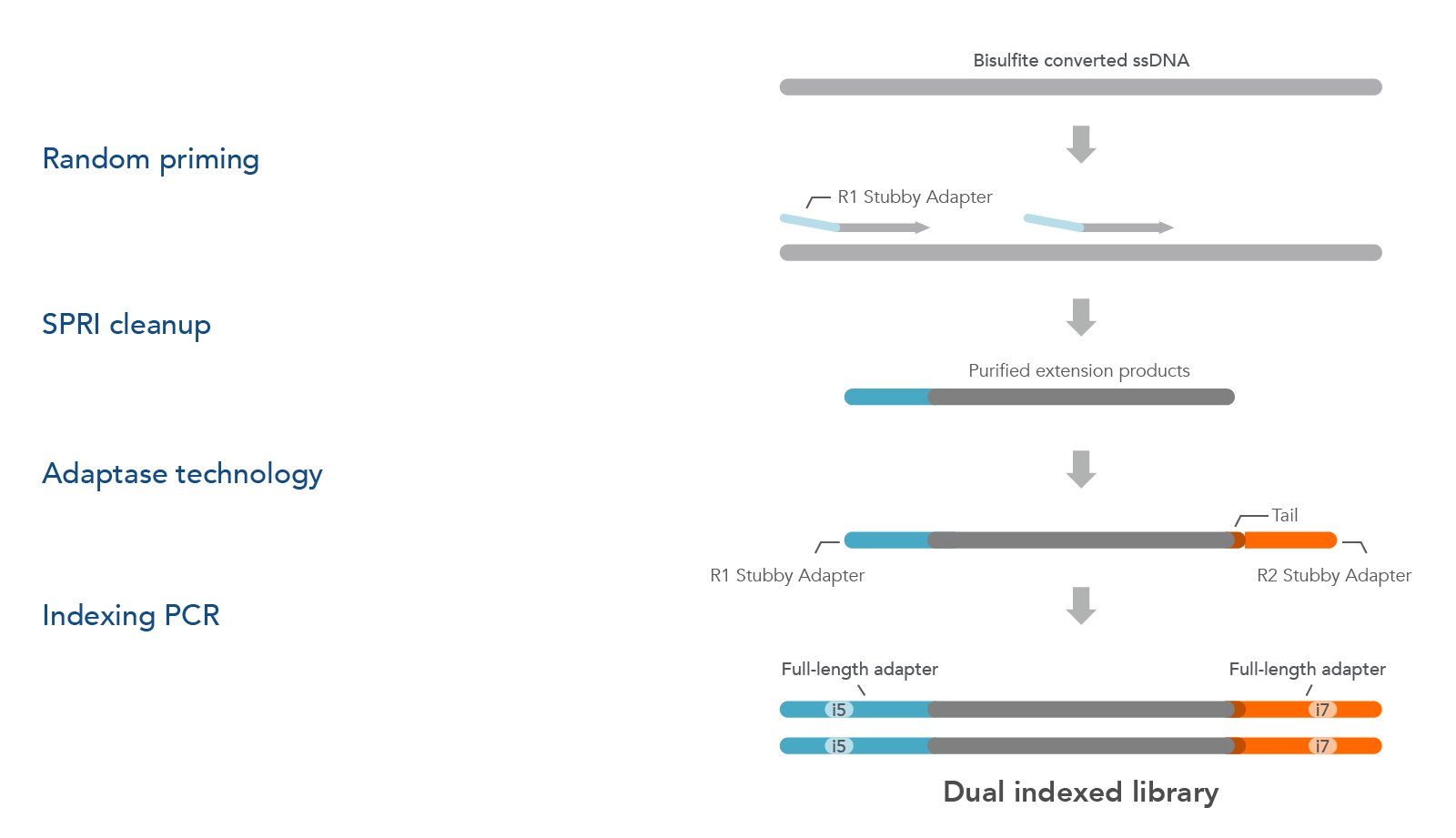
xGen Adaptase Module is also available to buy as a separate product (see ordering section). The xGen Adaptase Module supports a workflow that constructs NGS libraries from bisulfite-converted DNA from single cells. The xGen Adaptase Module maximizes the recovery of low concentrations of single-stranded DNA. In internal experiments, libraries created with this module have consistently exhibited higher complexity with reduced composition bias to provide a more faithful representation of the methylome. Using the in-line barcoding option, cells can be multiplexed to increase throughput and further reduce library prep costs.
The Adaptase Module only supplies the Adaptase reagents for the workflow (Figure 2). Random priming, purification, and indexing PCR reagents must be sourced separately. The Adaptase Module can be used for many applications, such as cataloging cell populations within heterogeneous tissues, assessing normal tissue for regulation of cellular mechanisms (e.g., differentiation), gaining insight into epigenetic alterations in disease states, and exploring across species to identify evolutionary conservation of epigenomic regulation.
Figure 2. The xGen Adaptase Module for single-cell bisulfite sequencing library preparation. First, bisulfite-converted ssDNA fragments are copied using custom random primers that are designed to incorporate the R1 Stubby Adapter (light orange) onto the 5´ end of the extension products using commercially available enzymes and buffers. Random primers can be designed to incorporate in-line barcode sequences for downstream multiplexing. During the next step, the proprietary xGen Adaptase reagent simultaneously tails and ligates the R2 Stubby Adapter (light blue) to the 3´end of the fragments. To complete the library, library amplification with indexing primers (not part of the xGen Adaptase Module) creates dual indexed library fragments for Illumina sequencing instruments.
NOTE: To support the workflow above, custom oligonucleotide primers and commercially available enzymes and buffers for complete functionality are sourced separately. Please see the xGen Adaptase Module for Single-Cell Methyl-Seq Library Preparation protocol for a complete list of required materials.
For product data on the individual xGen Adaptase Module, please review [2] or [3]
Product data
Methyl-seq library preparation workflow comparisons
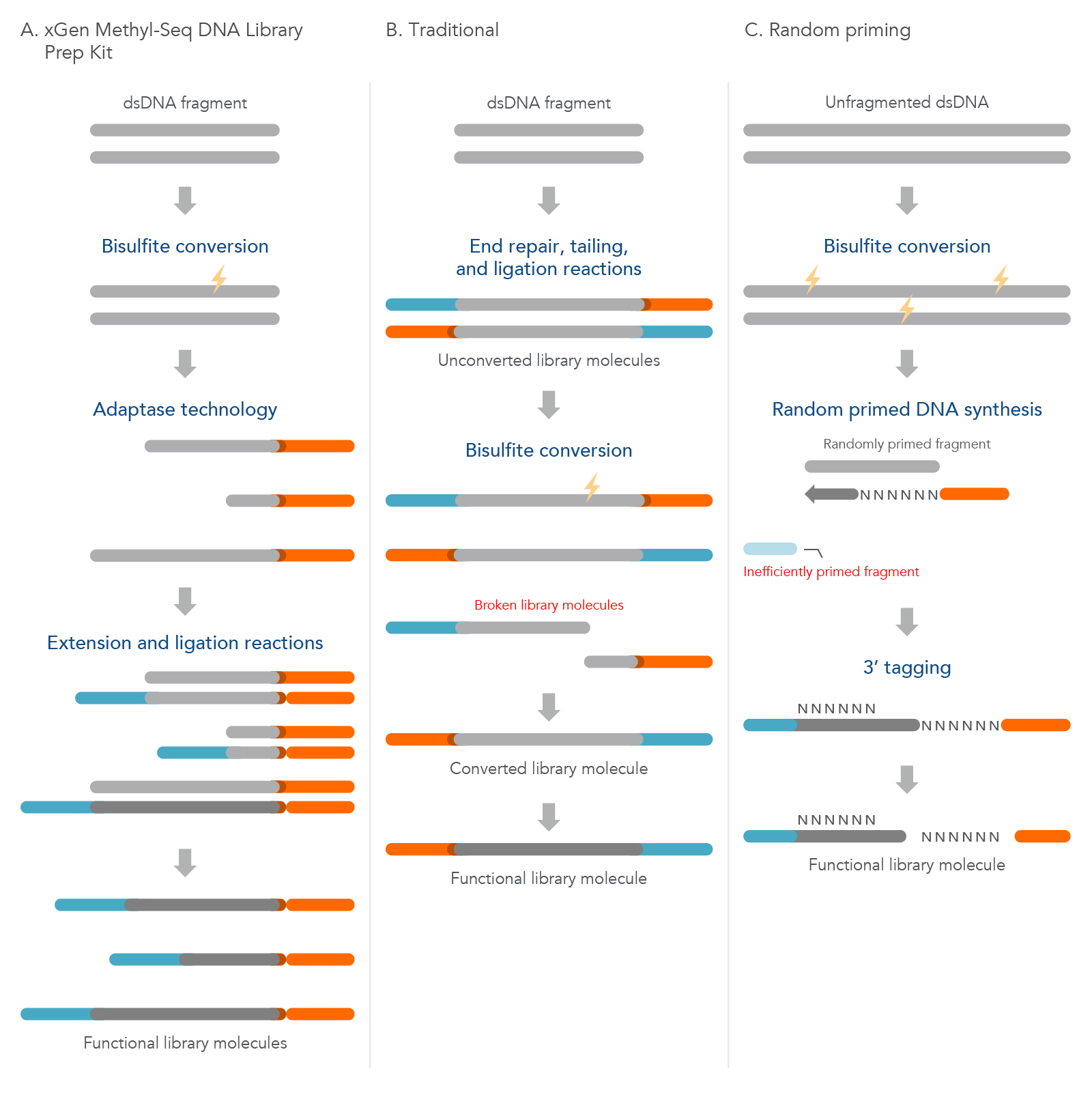
The xGen Methyl-Seq DNA Library Prep Kit has a post-bisulfite library preparation workflow to maximize library complexity by converting bisulfite-induced single-stranded fragments directly into library molecules. Traditional methods construct libraries from dsDNA using methylated adapters followed by bisulfite conversion that leads to significant library loss due to bisulfite-induced DNA fragmentation. The random priming methods are also post-bisulfite but have reduced library complexity due to a lower efficiency biased workflow (see Table 2; Figure 3).
Figure 3. Comparison of the xGen Methyl-Seq DNA Library Prep Kit workflow to two other common methods. (A) Before using the xGen Methyl-Seq DNA Library Prep Kit, double-stranded DNA (dsDNA) fragments undergo bisulfite conversion. The kit then directly converts these fragments into library molecules as described previously. (B) The traditional method of methylation sequencing builds the library before bisulfite conversion, which can lead to broken library molecules. (C) The random priming method converts unfragmented dsDNA with bisulfite treatment first, but due to inefficient random priming, many library molecules are lost and are not represented in the final library.
Library complexity and coverage from 1 ng of input DNA
To demonstrate uniform and comprehensive genome coverage using the xGen Methyl-Seq DNA Library Prep Kit, a titration experiment using 100 ng, 10 ng, or 1 ng of Arabidopsis thaliana genomic DNA was performed in duplicate and compared to two alternative methods (random primer and traditional) using these same input DNA samples. Each A. thaliana file was normalized to 30.2 million reads and data reported as an average of duplicate bisulfite-converted samples.
The xGen Methyl-Seq DNA Library Prep Kit demonstrates a higher, more comprehensive genome coverage and lower duplication rates than both alternative methods for each of the input samples—even the lowest concentration of 1 ng Arabidopsis DNA. The traditional method had equivalent results at the 100 ng input but lost significant complexity with the lower inputs. In contrast, the random-primed method had lower complexity and higher duplicate reads for each input concentration.
Table 2. Comparison of NGS library metrics obtained using the xGen Methyl-Seq DNA Library Prep Kit to traditional or random-primed methylation sequencing workflows.
| Input | Sample | % Reads aligned | Genome coverage | % Duplicate reads | Est. library size (Millions) | % CpX missing | % CpX covered ≥ 10X |
|---|---|---|---|---|---|---|---|
| 100 ng Arabidopsis | xGen Methyl-Seq | 89.6 | 22.0X | 1.9 | 714 | 0.56 | 92.2 |
| Traditional | 80.2 | 21.0X | 2.7 | 604 | 0.57 | 88.1 | |
| Random Primer | 71.4 | 16.0X | 22.1 | 48 | 7.70 | 39.4 | |
| 10 ng Arabidopsis | xGen Methyl-Seq | 87.8 | 22.0X | 2.7 | 406 | 0.58 | 90.4 |
| Traditional | 76.7 | 19.0X | 11.9 | 70 | 0.57 | 83.9 | |
| Random Primer | 71.9 | 16.0X | 22.2 | 45 | 5.2 | 45.2 | |
| 1 ng Arabidopsis | xGen Methyl-Seq | 83.3 | 18.0X | 18.2 | 38 | 0.59 | 77.1 |
| Traditional | 80.7 | 10.0X | 62.3 | 6 | 2.00 | 17.0 | |
| Random Primer | 73.4 | 12.0X | 46.1 | 12 | 6.60 | 31.3 | |
| 10 ng Human | xGen Methyl-Seq | 86.4 | 8.9X | 7.9 | 1,393 | N/A | N/A |
Resources
Frequently asked questions
What are the new names of the Swift products?
The Swift products were rebranded and now belong to the xGen™ NGS product line. The following lists the old Swift product name, and then the new name hyperlinked to its current product page.
| Swift product name | New IDT product name |
|---|---|
| Accel-NGS® Adaptase® Module for Single-Cell Methyl-Seq | xGen Adaptase™ Module |
| Normalase® Amplicon Panels (SNAP) Core | xGen Amplicon Core |
| SARS-CoV-2 Additional Genome Coverage Panel | xGen SARS-CoV-2 Expanded Amplicon Panel |
| SARS-CoV-2 S Gene Panel | xGen SARS-CoV-2 SGene Amplicon Panel |
| SNAP Set 1A Combinatorial Dual Indexing Primers | xGen Amplicon CDI Primers |
| Swift Combinatorial Dual Indexing Primers | xGen CDI Primers |
| Swift Normalase® Combinatorial Dual Indexing Primers | xGen Normalase CDI Primers |
| Accel-NGS 1S Plus DNA Library | xGen ssDNA & Low-input DNA Library Prep |
| Swift 2S Sonic DNA Library | xGen DNA Library Prep MC |
| Swift 2S Sonic Flexible DNA Library | xGen DNA Library Prep MC UNI |
| Swift 2S Turbo v2 | xGen DNA Library Prep EZ |
| Swift 2S Turbo Flexible v2 DNA Library | xGen DNA Library Prep EZ UNI |
| Accel-NGS Methyl-Seq DNA Library | xGen Methyl-Seq Library Prep |
| Swift Normalase | xGen Normalase Module |
| Swift Rapid RNA Library | xGen RNA Library Prep Kit |
| Swift RNA Library | xGen Broad-Range RNA Library Prep |
Why is my methylation sequencing run exhibiting low Q-scores?
Bisulfite conversion results in low complexity libraries since they are C-depleted, which may require a high complexity spike-in such as PhiX library to avoid low Q-scores.
Contact Illumina if you need specific recommendations on sequencing bisulfite converted samples for your sequencing instrument.
Which bisulfite conversion kits are compatible with the xGen™ Methyl-Seq DNA Library Prep Kit?
The xGen Methyl-Seq DNA Library Prep Kit has been evaluated with the EZ DNA Methylation-Gold™ Kit (Zymo Research).
If using the plate format, be sure to follow centrifugation recommendations as insufficient centrifugal force (x g) will result in poor recovery. Also, for the best results, do not extend the desulpheration incubation time.
Is the xGen™ Methyl-Seq DNA Library Prep Kit compatible with reduced representation bisulfite sequencing (RRBS)?
Yes.
The chemistry of xGen Methylation Sequencing easily works in an RRBS workflow. To capture the fraction of DNA fragments that are enriched for CpG islands, this RRBS protocol requires that size selection of inserts occurs after MspI DNA digestion, but before bisulfite conversion.
As the xGen Methyl-Seq DNA Library Prep Kit requires bisulfite conversion prior to library preparation, size selection must be performed on the DNA fragments immediately following MspI digestion (rather than on library molecules, as in the traditional workflow).
Does the Adaptase™ tail need to be bioinformatically trimmed for methylation sequencing?
Yes.
Bases added to the 3’ termini of DNA fragments during the Adaptase reaction contain unmethylated cytosines, which adds both artifactual sequence and methylation information to the dataset. Therefore, tail trimming of xGen™ Methylation-Sequencing DNA libraries is required to attain sufficient mapping efficiency and precise methylation information.
See our technical note, Tail Trimming for Better Data for further details on the tail and how to trim it.
Can the xGen™ Methyl-Seq DNA Library Prep Kit be used with ancient DNA?
Yes.
Many users are attracted to this technology because it can convert the short, single-stranded fragments common in ancient DNA into NGS library molecules.
Additionally, xGen Methyl-Seq DNA Library Prep Kit is capable of preserving uracil-containing DNA resulting from the damage inflicted on ancient DNA samples.
Contact us for recommended changes to the standard protocol.
Can bisulfite conversion replace the Fragmentation Step in the xGen™ Methyl-Seq workflow?
It is not recommended.
Fragmentation solely by bisulfite conversion results in a broad size distribution of larger DNA fragments. Such fragments may be converted into library molecules. However, the wide distribution of library molecules will cluster poorly on the flow cell. If skipping fragmentation is unavoidable, we recommend examining the size of library molecules prior to library quantification, and, if necessary, performing a right-side size selection to remove very large library molecules to improve clustering on the flow cell.
This scenario will result in reduced complexity of sample representation.
Contact us for recommended modifications when relying on bisulfite treatment for fragmentation.
References
- Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012 May 29;13(7):484-92. doi: 10.1038/nrg3230. PMID: 22641018.
- Luo C, Keown CL, Kurihara L, et al. Single-cell methylomes identify neuronal subtypes and regulatory elements in mammalian cortex. Science (New York, NY). 2017;357(6351):600-604.
- Luo C, Rivkin A, Zhou J, et al. Robust single-cell DNA methylome profiling with snmC-seq2. Nat Commun. 2018;9(1):3824.