Move over, axolotl—there’s a new longest-genome winner in the animal kingdom
When it comes to genome length in animals, here are a few interesting facts. The animal with the smallest genome is the Carsonella ruddi, which has 160,000 base pairs of DNA. Chickens have about 1 billion, while dogs clock in with around 2.2 billion, and humans have 3 billion. Science’s most-studied genome belongs to the fruit fly, which has 139.5 million base pairs.
For several years, it was believed that the longest genome in the animal world belonged to the axolotl. The axolotl, also known as the Mexican walking fish, is a type of salamander found in lakes in and around Mexico City.
Axolotls were once a staple in the Aztec diet but faced extinction in the wild by 2010 due to urbanization, water pollution, and invasive species. They have also been the subject of considerable scientific investigation, and large numbers are bred in captivity. A study in 2018 pegged its genome length at 32 billion base pairs and revealed species-specific genetic pathways that could have been responsible for the animal’s limb regeneration abilities.
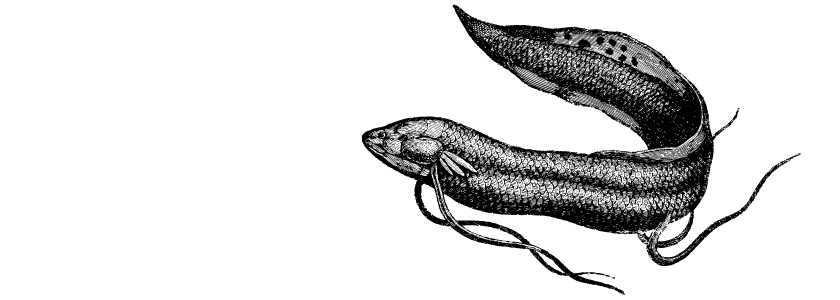
But the axolotl genome is now old news—in January, a team of researchers crowned a new long-genome winner for the animal kingdom: the Australian lungfish, which was found to have a 43-billion-base-pair genome.
The lungfish’s genome was sequenced using more than 72 million reads. Researchers developed dedicated software to assemble the genome from those millions of pieces; they hope the findings will serve as a resource for the study of regenerative tissue. The reads were sequenced at the DRESDEN Concept Genome Center in Germany, with software systems developed by a team in Heidelberg, Germany, and financing for the sequencing machines courtesy of the Klaus Tschira Foundation and the Max Planck Society.
Sequencing advancements produce a new long-genome champion
Writing in Nature, researchers reported that the Australian lungfish, Neoceratodus forsteri, had a genome that was some 30% larger than the axolotl’s and 14 times bigger than our own.
The Australian lungfish—one of just six types of the species—can grow up to 22 pounds and 4 feet in length. Called a living fossil, the species has not changed for more than 100 million years. The animal is nocturnal, carnivorous, has complex courtship behavior, and females do not breed until they are about 22 years old. Its native habitat is two rivers in the lowland sugar-growing parts of the Australian state of Queensland, north of Brisbane. Australian lungfish are reported to live their entire lives in a single pool, or occasionally two adjacent pools, and individuals can apparently live out of water for several days at a stretch if its skin stays moist.
The lungfish sequencing—which was carried out by the same team that did the axolotl sequencing—is significant for two reasons: the achievement of sequencing such a huge genome opens the door for future, more complex sequencing. Knowledge about the lungfish helps researchers better understand how fish sprouted legs and crawled out of the water to live on land.
The long genome, researchers wrote, was due to vast intergenic regions and introns with a lot of repeating content—about 90%--and it illustrates how vertebrates moved on to land.
“Preadaptations to living on land include gaining of limb-like expression of developmental genes such as hoxc13 and sall1 in their lobed fins,” researchers wrote. “Increased rates of evolution and duplication of genes associated with obligate air-breathing such as lung surfactants and the expansion of odorant receptor gene-families that detect airborne odors contribute to their tetrapod-like biology.”
Though huge, the lungfish genome is in line with the genome of the fern Tmesipteris obliqua and the flowering plant Paris japonica, which grows in alpine regions of Japan and has a genome stretching to 148.8 billion base pairs.
Is there an upper limit to genome length?
Which brings up an interesting question: just how long could genomes be?
Gene lengths correlate with gene duplication and alternative splicing, noted a 2014 paper in Genome Research, and longer genes are less likely to produce duplicates and more likely to show splicing. But gene length, the paper added, is dynamic, increasing with time due in part to insertions of transposable elements and decreasing after partial gene duplication.
“Gene length and gene expression level are associated with both a gene’s family size and its number of splice variants,” the paper summarized. “Together, gene length and gene expression level can account for the relationship between gene duplication and alternative splicing.”
Eukaryote genomes range in size by a factor of 64,000, found a study in Cell Press, but the parts making up genes, regulatory regions, and other functional areas are just a fraction of total genome size—most of the range is due to repetitive, parasitic, and “often selfishly accumulating DNA and their degraded products. Despite the diversity, most species have small genomes, and those with giant genomes are the exception and belong to only a few phylogenetically distinct lineages.”
But will genomes go higher than the lungfish’s? Probably not by much, the report said.
“The synthesis of available information on giant genomes suggests that the biological limit to genome size expansion in eukaryotes may have been reached,” the authors wrote.
One possible reason could be that there are clear biochemical costs and biological consequences as DNA size increases, including increased risk of extinction in plants and reduced brain complexity in animals.
“Giant genomes are predicted to arise in circumstances where the negative impact of genome obesity is less severe and/or the selective pressure more ‘permissive,’” indicated an article in Botanical Journal of the Linnean Society. The authors suggested that genome expansion in some cases could be due to the presence of storage organs like bulbs and rhizomes that provide nutritional and energetic independence of the plant from its external environment.
Research reveals that lungfish are the closest living fish relatives of humans
Sequencing of the lungfish helped answer a nagging evolutionary question: who is more closely related to land-dwelling animals—the lungfish or the coelacanth? Now we know that coelacanths diverged first, with lungfish diverging to form four-legged animals about 420 million years ago.
The Australian lungfish has a lot in common with fish, but has evolved some characteristics of terrestrial vertebrates, and “are the closest living fish relatives of humans,” states Phys.org. The bones in their fins resemble tetrapods, including human limbs, and they move more like a salamander than a fish.
Expect more giant genomes, say the authors of the lungfish study. Groundbreaking work began with the axolotl and perfected with the lungfish to pave the way for more discoveries. Not only did this study offer lessons about how life adapted to land, but it may also explain why some genomes evolve to be so huge.
“To overcome the challenges posed by the lungfish genome, we had to further develop and adapt the methods that had yielded the axolotl genome sequence three years ago,” said Siegfried Schloissnig, a computer scientist and biologist at the Research Institute of Molecular Pathology at the Vienna BioCenter, and one of the study’s contributors. “Our algorithm is now applicable to any other genome and opens the door for new challenging sequencing projects.”


 Processing
Processing
